Formic Acid
CAS : 64-18-6
Formic Acid
DESCRIPTION
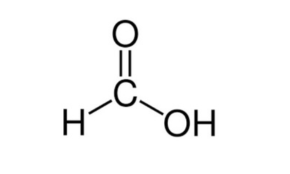
Formic acid, systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula H2CO2. It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts, and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol.
PROPERTIES
| Appearance | Colorless fuming liquid |
| Odor | Pungent, penetrating |
| Density | 1.220 g/mL |
| Melting point | 8.4 °C (47.1 °F; 281.5 K) |
| Boiling point | 100.8 °C (213.4 °F; 373.9 K) |
| Solubility in water | Miscible |
| Solubility | Miscible with ether, acetone, ethyl acetate, glycerol, methanol, ethanol Partially soluble in benzene, toluene, xylenes |
APPLICATION
The principal use of formic acid is as a preservative and antibacterial agent in livestock feed. When sprayed on fresh hay or other silage, it arrests certain decay processes and causes the feed to retain its nutritive value longer, and so it is widely used to preserve winter feed for cattle. In the poultry industry, it is sometimes added to feed to kill salmonella bacteria. Other uses:
· It is used to process organic latex (sap) into raw rubber.
· Beekeepers use formic acid as a miticide against the Varroa mite.
· It is of minor importance in the textile industry and for the tanning of leather.
· Some formate esters are artificial flavorings or perfumes.
· It is the active ingredient in some brands of household limescale remover.
PACKAGING OPTIONS
Carboy, Drums
Enquiry Now