Acetone
CAS : 67-64-1
Acetone
DESCRIPTION
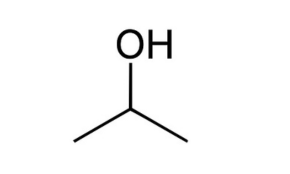
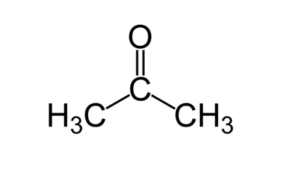
Acetone (systematically named propane) is an organic compound with the formula (CH3)2CO. It is a colorless, volatile, flammable liquid, and is the simplest ketone.
PROPERTIES
| Physical State And Appearance | Liquid |
| Odor | Fruity, mint-like. Fragrant, Ethereal |
| Molecular Weight | 58.08 g/mole |
| Color | Colorless. Clear |
| Boiling Point | 56.2°C (133.2°F) |
| Critical Temperature | 235°C (455°F) |
| Flash Point | −17 °C |
| Melting Point | -95.35 (-139.6°F) |
| Specific Gravity | 0.79 (Water = 1) |
| Vapor Pressure | 24 kappa (@ 20°C) |
| Vapor Density | 2 (Air = 1) |
| Odor Threshold | 62 ppm |
| Water/Oil Dist. Coif | The product is more soluble in water; log(oil/water) = - 0.2 |
| Dispersion Properties | See solubility in water |
| Solubility | Easily soluble in cold water, hot water |
| Acetone wt.% | 94 min |
| MEK, wt.% | 2.0 max |
| MIPK (methyl isopropyl ketone) wt.% | 0.5 max |
| Water, wt.% | 3.0 max |
| Specific Gravity @ 20/20C wt.% | 0.82 max |
| Non-volatile Residue wt.% | 0.001 max |
APPLICATION
· Acetone is a good solvent for most plastics and synthetic fibres including those used in laboratory bottles made of polystyrene, polycarbonate and some types of polypropylene. It is used as a volatile component of some paints and varnishes.
· Although flammable itself, acetone is also used extensively as a solvent for the safe transporting and storing of acetylene, which cannot be safely pressurized as a pure compound.
· Acetone is used in a variety of general medical and cosmetic applications and is also listed as a component in food additives and food packaging. Acetone is commonly used in the skin rejuvenation process in medical offices and medical spas. Since the days of ancient Egypt, people have been using chemexfoliation methods, also known as chemical peeling, to rejuvenate skin.
· In the laboratory, acetone is used as a polar aprotic solvent in a variety of organic reactions, such as SN2 reactions. The use of acetone solvent is also critical for the Jones oxidation. It is a common solvent for rinsing laboratory glassware because of its low cost and volatility; however, it does not form a zoetrope with water.
· It can be used as an artistic agent; when rubbed on the back of a laser print or photocopy placed face-down on another surface and burnished firmly, the toner of the image transfers to the destination surface.
PACKAGING OPTIONS
Tanks,
Drums
Enquiry Now