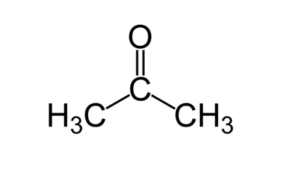
Acetonitrile
CAS : 75-05-8
Acetonitrile
DESCRIPTION
Acetonitrile is the chemical compound with the formula C2H3N. This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene
PROPERTIES
| Appearance | Transparent liquid, no visible impurities |
| Chromaticity (in Hazen) (Pt.-Co) ≤ | <5 |
| Moisture % (m/m) ≤ | 0.0049 |
| Hydrocyanic Acid % (mg/kg) ≤ | 3.6 |
| Acetone (mg/kg) ≤ | < 5.0 |
| Acrylonitrile % ≤ | < 5.0 |
| Heavy Component (including prop nitrile) % ≤ | 136.0 |
| Purity % ≥ | 99.98 |
| Ammonia (mg/kg) ≤ | 3.22 |
| Acidity (mg/kg) ≤ | 28.0 |
| Density (at 20°C) g/cm 3 | 0.781 |
| Copper (mg/kg) ≤ | 0.01 |
| Iron (mg/kg) %≤ | 0.04 |
| Boiling Range (Under 0.10133MPa) ° C | 81.1~81.8 |
APPLICATION
· Acetonitrile is primarily used as an extraction solvent for butadiene.
· It is used as a chemical intermediate in pesticide manufacturing; and as a solvent for both inorganic and organic compounds.
· It is also included as a starting material for the production of acetophenone, alpha- naphthalenacetic acid, thiamine, and acetamidine; to remove tars, phenols, and coloring matter from petroleum hydrocarbons not soluble in Acetonitrile.
· In the laboratory, acetone is used as a polar aprotic solvent in a variety of organic reactions, such as SN2 reactions. The use of acetone solvent is also critical for the Jones oxidation. It is a common solvent for rinsing laboratory glassware because of its low cost and volatility; however, it does not form a zoetrope with water.
· It is used in the production of acrylic fibers; and in pharmaceuticals, perfumes, nitrile rubber, and ABS (Acrylonitrile-butadiene-styrene) resins.
PACKAGING OPTIONS
Drums
Enquiry Now